Oil drop experiment
In the famous oil drop experiment, Millikan and Fletcher determined the charge of the electron. In the experiment, small drops of oil were sprayed into a chamber and observed through a microscope as they fell through the air.
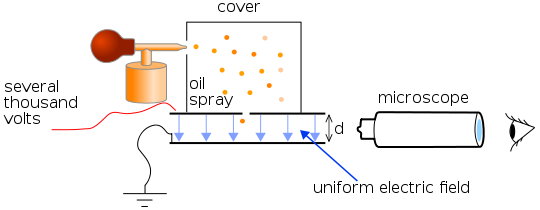
A schematic diagram of the experiment. Source: Wikimedia Commons.
Small drops quickly achieve terminal velocity as the downward gravitational force is balanced by the air drag force. For small drops, the drag force can be described by Stokes's law,
\[ \begin{equation} \large F_{\text{drag}}=6\pi \eta rv. \end{equation} \]Here $\eta = 1.81 \times 10^{-5}$ [kg/s m] is the viscosity of air, $r$ is the radius of the oil drop, and $v$ is the downwards velocity. At terminal velocity, the drag force is balanced by the downward gravitational force and the upwards bouancy force.
\[ \begin{equation} \large 6\pi \eta rv = \frac{4 \pi r^3}{3}(\rho_{\text{oil}}-\rho_{\text{air}})g. \end{equation} \]Here $\rho_{\text{oil}}=900$ [kg/m³] and $\rho_{\text{air}}= 1.2922$ [kg/m³] are the densities of oil and air, and $g=9.81$ [m/s²] is acceleration of gravity. By observing the terminal velocity of a drop it is possible to determine its radius.
\[ \begin{equation} \large r= \sqrt{\frac{9 \eta v}{ 2(\rho_{\text{oil}}-\rho_{\text{air}})g}}. \end{equation} \]There were two metal plates in the chamber that could be used to apply an electric field in the vertical direction. The oil drops were randomly charged and by applying an electric field that balances the gravitational and bouancy forces, a drop can be suspended motionless in the air. Under those conditions, the electric force balances the gravitational and bouancy forces,
\[ \begin{equation} \large qE= \frac{4 \pi r^3}{3}(\rho_{\text{oil}}-\rho_{\text{air}})g. \end{equation} \]Here $q$ is the charge of the drop and $E$ is the electric field. Using the expression for the radius of the drop, this can be written,
\[ \begin{equation} \large q=\frac{18 \pi (\eta v)^{3/2}}{E\sqrt{2(\rho_{\text{oil}}-\rho_{\text{air}})g}}=3.28\times 10^{-8}\frac{v^{3/2}}{E}. \end{equation} \]This allowed them to measure the charge on the drops. They discovered that the charge was quantized in units of $1.6022\times 10^{-19}$ C.
The oil drop experiment is simulated below. The oil drops have random sizes and charges. Some are positively charged and some are negatively charged. It the real experiment, the drops are so small that you cannot determine their size but it is possible in the simulation to show the size and charge of the particles. To measure the charge on a drop, adjust the electric field to try to keep a drop motionless and record the electric field. Then turn the electric field off and observe how fast the drop falls. The divisions in the graph are 0.1 mm. From this information and the equations above, it is possible to calculate the charge.