First name
Last name
Matrikelnr.
Problem 1
Consider a molecule formed by a lithium atom and a hydrogen atom. The lithium atom consists of three protons (and four neutrons) in the atomic nucleus (mass $m_{\text{Li}}$ at position $\vec{r}_{\text{Li}}$) paired with three electrons (mass $m_e$ at positions $\vec{r}_1$, $\vec{r}_2$, $\vec{r}_3$) in the 1s state and 2s state. The hydrogen atom has one proton in its atomic nucleus (mass $m_p$ at position $\vec{r}_p$) paired with one electron (mass $m_e$ at position $\vec{r}_4$) in the 1s shell. You may use sums over the electron positions $\vec{r}_i$ such as $\sum\limits_{i=1}^4$.
a) Write down the many particle Hamiltonian for the LiH molecule. Please use the proper masses ($m_{\text{Li}}$, $m_p$, $m_e$), their charges ($\pm e$, $+3e$) and their positions ($\vec{r}_{\text{Li}}$, $\vec{r}_p$, $\vec{r}_1$, $\vec{r}_2$, $\vec{r}_3$, $\vec{r}_4$) of the involved particles.
b) Write down the molecular orbital Hamiltonian for a single electron for the molecule LiH. How many terms does this Hamiltonian consists of? Neglect Slater rules for the effective nuclear charge.
c) A molecular orbital wavefunction (MO-wavefunction) can be constructed for the Hamiltonian given in b) by a linear combination of atomic orbitals. Write down an Ansatz for the MO-wavefunction using atomic orbitals. Please use exact notation for the used wavefunctions.
d) How many molecular orbitals would be obtained by using the Ansatz MO-wavefunction you gave in part (c)?
Problem 2
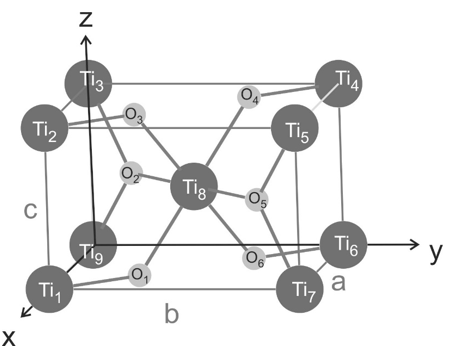
Rutile is one crystalline phase of titanium dioxide which crystallizes in a tetragonal structure ($a = b = 4.594$ Å, $c = 2.959$ Å, and $\alpha = \beta = \gamma = 90$°). A sketch of the crystallographic unit cell is given below with dark grey spheres as titanium atoms and light grey spheres as oxygen atoms. The titanium atoms are located at the edges and one at the center of the unit cell, two oxygen atoms are in the $a,b$ planes and two oxygen atoms are inside of the unit cell.

a) How many titanium atoms and how many oxygen atoms are within the crystallographic unit cell?
b) The tetragonal unit cell is a primitive Bravais lattice (one lattice point per unit cell). Which atoms are the basis of the unit cell? Please denote the atoms with their respective labels (Ti1, Ti2, O1, O2, etc..)
c) Draw the crystallographic plane with the Miller indices $h = 2$, $k = 0$, $l = 3$. Use the sketch below.
d) Give the interplanar distance $d_{hkl}$ of the (203) planes.

Problem 3
a) Give the reciprocal space vectors for rutile, the lattice constants are given in problem 2. Do not forget to give units.
b) Diffraction should be observed of the 201 reflection by using X-rays with a wavelength of 1.54 Å. Draw the reciprocal lattice vector $\vec{G}_{201}$ in the $k_x-k_z$ plane and the incident X-ray beam (with symbol $\vec{k}$) and of the scattered X-ray beam (with symbol $\vec{k}'$) for observation of the 201 diffraction peak. Please use a ruler, a free hand sketch will not be accepted.