 |
Problem 1
The element Sn is a metal above 13° C and a semiconductor below 13° C.
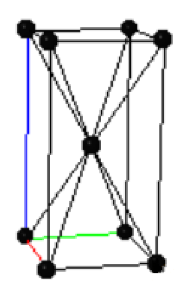
(a) The metallic phase has a body-centered tetragonal Bravais lattice with two atoms in the primitive unit cell. The conventional unit cell of the metallic phase is shown on the right, where the black points are the Bravais lattice points. How many atoms are there in the conventional unit cell? Explain your reasoning.
(b) The semiconducting phase has an fcc Bravais lattice with two atoms in the primitive unit cell. The Miller indices are given in terms of the simple cubic conventional unit cell. Give the Miller indices of a direction that points from one Bravais lattice point to a nearest neighbor Bravais lattice point.
(c) The semiconducting phase has a very small direct band gap at $\Gamma$. Draw the electron dispersion relation $E\text{ vs. }k$ for the semiconducting phase. Label the valence band, the conduction band, and the chemical potential.
(d) Which has more phonon modes, 10 grams of the metallic phase or 10 grams of the semiconducting phase? Explain your reasoning.
(e) Which has a higher specific heat at room temperature, the metallic phase or the semiconducting phase? Explain your reasoning.
Solution
Problem 2
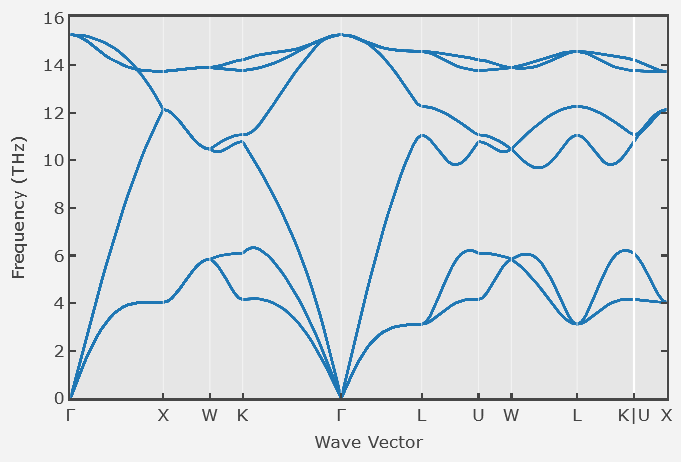
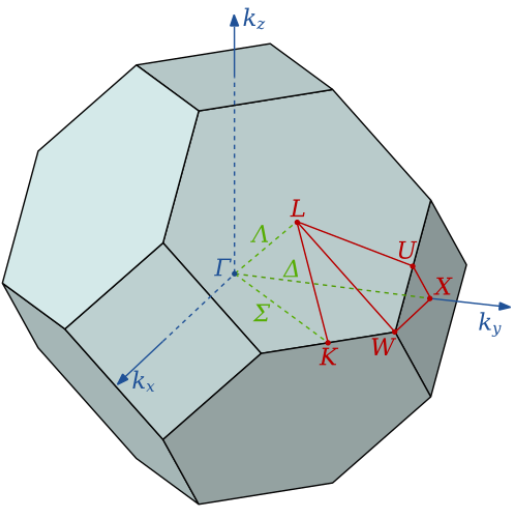
Below is the phonon dispersion relation for a crystal with an fcc Bravais lattice. The lattice constant is a = 0.2 nm.
 |  |
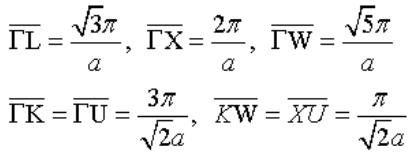 |
(a) How many atoms are there in the primitive unit cell?
(b) Choose a direction and a polarization (longitudinal or transverse) and estimate the speed of sound in this direction for long-wavelength sound waves.
(c) What is the shortest phonon wavelength possible in this crystal?
(d) What is the mean number of phonons in the phonon mode with the highest energy at 300 K?
Solution
Problem 3
(a) What information do you get in a single crystal x-ray diffraction experiment that cannot be obtained from a powder diffraction experiment?
(b) In a single crystal x-ray diffraction experiment, how do you determine the Bravais lattice of a crystal?
(c) How are the Brillouin zone boundaries defined?
(d) What is an Ewald sphere? What is the radius of an Ewald sphere?
Solution
Problem 4
State whether the following materials are metals, semiconductors, or insulators. Briefly explain your reasoning in each case. It can happen that you cannot tell if a material is a semiconductor or an insulator. Write semiconductor/insulator in this case, and explain why you can't tell.
(a) The specific heat at low temperatures is proportional to temperature. There is an electron contribution to the specific heat and a phonon contribution to the specific heat to consider.
(b) The material is transparent to visible light. Photons are not absorbed if the photon cannot lift an electron from an occupied state to an empty state.
(c) The electrical conductivity improves dramatically when infrared light shines on this material. The electrical conductivity depends on the electron density.
(d) The electron density of states at the chemical potential is zero.
(e) A one-dimensional crystal with three valence electrons per unit cell. Consider a 1D band structure and fill the bands.
(f) The electrical conductivity increases as temperature increases. The electrical conductivity depends on the electron density.
(g) The phonon contribution to the internal energy density dominates at room temperature.
Solution